 In the US, goods in the food and beverage and pharmaceutical industries must adhere to FDA 21 CFR Part 11 regulations. These regulations are guidelines that specify what information is required in order to leave an audit trail of records. This information generally includes electronic signatures that detail things like user activity on the machine, recipes, and reports that are generated at the end of each project. In order for a machine to help maintain compliance with 21 CFR Part 11, it’s important that all necessary data is logged automatically.
In the US, goods in the food and beverage and pharmaceutical industries must adhere to FDA 21 CFR Part 11 regulations. These regulations are guidelines that specify what information is required in order to leave an audit trail of records. This information generally includes electronic signatures that detail things like user activity on the machine, recipes, and reports that are generated at the end of each project. In order for a machine to help maintain compliance with 21 CFR Part 11, it’s important that all necessary data is logged automatically.
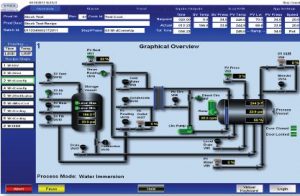 InduSoft is now standard the standard development solution for the ICONRMS system. InduSoft was able to address the basic requirements of FDA 21 CFR Part 11 compliance, as well as include other tools that made developing the HMI of the retort and sterilization machines easy. The system that Stock America developed uses InduSoft to connect to Allen Bradley CompactLogix and Allen Bradley SLC 5/05 PLCs using native drivers that areincluded standard with InduSoft Web Studio.
InduSoft is now standard the standard development solution for the ICONRMS system. InduSoft was able to address the basic requirements of FDA 21 CFR Part 11 compliance, as well as include other tools that made developing the HMI of the retort and sterilization machines easy. The system that Stock America developed uses InduSoft to connect to Allen Bradley CompactLogix and Allen Bradley SLC 5/05 PLCs using native drivers that areincluded standard with InduSoft Web Studio.
They have the ability to easily develop HMIs for their machines that make FDA and USDA compliancy hassle-free.
This is an InduSoft application success story.
Tri-Phase Automation is an InduSoft distributor in Wisconsin
Contact Tri-Phase Sales or Get A Quote
